Growth-Promoting Implants for Beef Cattle
Growth-promoting implants offer beef cattle producers a safe and effective way to increase calf weight gains. Implants increase production of muscle tissue and often reduce body fat production. This results in significant improvements in both growth rate and feed efficiency. Despite the proven benefits of implant use, only 11.9 percent of beef cattle operations surveyed by the National Animal Health Monitoring Service (NAHMS) implanted calves before or at weaning.
When used properly, growth-stimulating implants can enhance average daily gain in suckling calves by 4 to 8 percent, in growing calves by 10 to 20 percent, and in finishing cattle by 15 percent. In addition, feed efficiency is expected to improve by 6 to 8 percent in growing cattle and by 8 to 10 percent in finishing cattle.
Adequate nutrition is needed for an implant to enhance calf growth performance. Implants will not make up for poor nutrition. Calf gains must be at least 1.3 pounds per day for implants to be effective at improving growth. Expected return on investment for implant use under proper management is often 10 to 1.
Implants are available for sucking, stockering, and finishing phases of beef cattle production. While implanting may be beneficial for an individual production phase, it is important to consider implant impacts on later production phases, particularly with respect to marketing and retained ownership. Implant effects on quality grade and palatability of the end product must be considered. In some instances, aggressive implanting protocols can reduce quality grade of beef end products. Responsible and strategic implanting programs can make best use of implants while maintaining acceptable end-product quality. For instance, altering the timing of implant administration in relation to harvest can reduce the effects of implanting on quality grade.
Available Implants
The U. S. Food and Drug Administration (FDA) approves and regulates the use of growth-promoting implants for beef cattle. Implants should be administered only in the FDA-approved location in beef cattle, which is between the skin and cartilage in the middle one-third of the backside of the ear.
Implants are typically small pellets impregnated with specific growth promotants. Some implants also contain an antimicrobial, such as oxytetracycline or tylosin tartrate, to provide a local antibacterial effect. Implants are designed for sustained, slow release of the active ingredients and are administered under the skin (subcutaneously) on the backside of the ear midway between the ear tip and base.
Implants can be classified as either estrogenic (hormones affecting female characteristics) or androgenic (hormones affecting male characteristics), based on the specific growth promotants contained in the implants. Estradiol, progesterone, and zeranol are estrogenic. Androgenic implants often contain trenbolate acetate (TBA), which is chemically related to testosterone, alone or in combination with other active ingredients. Using only TBA in the final implant in feedlot cattle may reduce risk of animal health or carcass problems. When more than one implant was used, feedlots surveyed by NAHMS administered an androgenic implant as their final implant to most feedlot cattle.
|
Commercial Product Name |
Marketer |
Active Ingredient(s) |
Target Animal(s) |
Claim |
|
SYNOVEX C |
Pfizer Animal Health |
100 mg progesterone 10 mg estradiol benzoate |
Suckling beef calves up to 400 pounds; steers weighing more than 400 pounds and fed in confinement for slaughter when used as part of a re-implant program in which an initial Synovex C implant is followed at approximately 70 days by Synovex S; not for use in veal calves, calves less than 45 days old, or bull calves intended for reproduction |
Increase rate of weight gain |
|
SYNOVEX S |
Pfizer Animal Health |
200 mg progesterone 20 mg estradiol benzoate |
Steers weighing 400 pounds or more; for use in steers only; not for use in veal calves |
Increase rate of weight gain and improve feed efficiency |
|
SYNOVEX H |
Pfizer Animal Health |
200 mg testosterone priopionate 20 mg estradiol benzoate |
Nonreplacement heifers weighing 400 pounds or more; for use in heifers only; not for use in veal calves |
Increase rate of weight gain and improve feed efficiency |
|
SYNOVEX CHOICE |
Pfizer Animal Health |
100 mg trenbolate acetate 14 mg estradiol benzoate |
Steers fed in confinement for slaughter; for use in calf-fed and yearling feeding programs; not for use in veal calves |
Increase rate of weight gain |
|
SYNOVEX Plus |
Pfizer Animal Health |
200 mg trenbolate acetate 28 mg estradiol benzoate |
Steers and heifers fed in confinement for slaughter; not for use in veal calves |
Increase rate of weight gain and improve feed efficiency in steers; increase rate of weight gain in heifers |
|
RALGRO |
Intervet/ Schering-Plough Animal Health |
36 mg zeranol |
Suckling beef calves, including replacement heifers between 1 month of age and weaning, weaned beef calves, growing beef cattle, feedlot steers, and feedlot heifers; not for use in breeding herd replacements or lactating dairy cattle |
Increase rate of weight gain and improve feed efficiency |
|
RALGRO MAGNUM |
Intervet/ Schering-Plough Animal Health |
72 mg zeranol |
Steers fed in confinement for slaughter |
Increase rate of weight gain and improve feed efficiency |
|
REVALOR-200 |
Intervet/ Schering-Plough Animal Health |
200 mg trenbolate acetate 20 mg estradiol |
Steers fed in confinement for slaughter; not for use in breeding herd replacements or lactating dairy cattle; not for use in veal calves |
Increase rate of weight gain and improve feed efficiency |
|
REVALOR-G |
Intervet/ Schering-Plough Animal Health |
40 mg trenbolate acetate 8 mg estradiol |
Pasture cattle, including slaughter, stocker, and feeder steers and heifers; not for use in breeding herd replacements or lactating dairy cattle; not for use in veal calves |
Increase rate of weight gain |
|
REVALOR-H |
Intervet/ Schering-Plough Animal Health |
140 mg trenbolate acetate 14 mg estradiol |
Heifers fed in confinement for slaughter; not for use in breeding herd replacements or lactating dairy cattle; not for use in veal calves |
Increase rate of weight gain and improve feed efficiency |
|
REVALOR-IH |
Intervet/ Schering-Plough Animal Health |
80 mg trenbolate acetate 8 mg estradiol |
Heifers fed in confinement for slaughter; not for use in breeding herd replacements or lactating dairy cattle; not for use in veal calves |
Increase rate of weight gain |
|
REVALOR-IS |
Intervet/ Schering-Plough Animal Health |
80 mg trenbolate acetate 16 mg estradiol |
Steers fed in confinement for slaughter; not for use in breeding herd replacements or lactating dairy cattle; not for use in veal calves |
Increase rate of weight gain and improve feed efficiency |
|
REVALOR-S |
Intervet/ Schering-Plough Animal Health |
120 trenbolate acetate 24 mg estradiol |
Steers fed in confinement for slaughter; not for use in breeding herd replacements or lactating dairy cattle; not for use in veal calves |
Increase rate of weight gain and improve feed efficiency |
|
REVALOR-XS |
Intervet/ Schering-Plough Animal Health |
200 trenbolate acetate 40 mg estradiol |
Steers fed in confinement for slaughter; not for use in breeding herd replacements or lactating dairy cattle; not for use in veal calves |
Increase rate of weight gain and improve feed efficiency for up to 200 days |
|
FINAPLIX-H |
Intervet/ Schering-Plough Animal Health |
200 mg trenbolone |
Heifers fed in confinement for slaughter; not for use in breeding herd replacements or lactating dairy cattle; not for use in veal calves |
Increase rate of weight gain and improve feed efficiency |
|
COMPUDOSE |
Elanco Animal Health |
25.7 mg estradiol 0.5 mg oxytetracycline |
Suckling and pastured growing steers; finishing steers and heifers; not for use in breeding herd replacements; not for use in veal calves |
Increase rate of weight gain in suckling and pastured growing steers; increase rate of weight gain and improve feed efficiency in confined steers and heifers; effective daily dose of estradiol for at least 200 days |
|
ENCORE |
Elanco Animal Health |
43.9 mg estradiol 0.5 mg oxytetracycline |
Suckling and pastured growing steers; finishing steers and heifers; not for use in breeding herd replacements or dairy animals; not for use in veal calves |
Increase rate of weight gain in suckling and pastured growing steers; increase rate of weight gain and improve feed efficiency in confined steers and heifers; effective daily dose of estradiol for at least 400 days |
|
COMPONENT E-C2 |
Elanco Animal Health |
100 mg progesterone USP 10 mg estradiol benzoate |
Suckling beef calves up to 400 pounds; not for use in breeding herd replacements; not for use in veal calves, calves less than 45 days old, or bull calves intended for reproduction |
Increase rate of weight gain |
|
COMPONENT E-H2 |
Elanco Animal Health |
200 mg testosterone propionate USP 20 mg estradiol benzoate |
Heifers weighing 400 pounds or more; not for use in breeding herd replacements or dairy animals; not for use in veal calves |
Increase rate of weight gain and improve feed efficiency |
|
COMPONENT E-S2 |
Elanco Animal Health |
200 mg progesterone USP 20 mg estradiol benzoate |
Steers weighing 400 pounds or more; not for use in breeding herd replacements or dairy animals; not for use in veal calves |
Increase rate of weight gain and improve feed efficiency |
|
COMPONENT TE-G2 |
Elanco Animal Health |
40 mg trenbolate acetate 8 mg estradiol USP |
Pasture cattle including slaughter, stocker, and feeder steers and heifers; not for use in breeding herd replacements or dairy animals; not for use in veal calves |
Increase rate of weight gain |
|
COMPONENT TE-H2 |
Elanco Animal Health |
140 mg trenbolate acetate 14 mg estradiol USP |
Heifers fed in confinement for slaughter; not for use in breeding herd replacements or dairy animals; not for use in veal calves |
Increase rate of weight gain and improve feed efficiency |
|
COMPONENT TE-IH2 |
Elanco Animal Health |
80 mg trenbolate acetate 8 mg estradiol |
Heifers fed in confinement for slaughter; not for use in breeding herd replacements or dairy animals; not for use in veal calves |
Increase rate of weight gain |
|
COMPONENT TE-S2 |
Elanco Animal Health |
120 mg trenbolate acetate 24 mg estradiol |
Steers fed in confinement for slaughter; not for use in breeding herd replacements or dairy animals; not for use in veal calves |
Increase rate of weight gain and improve feed efficiency |
|
COMPONENT TE-IS2 |
Elanco Animal Health |
80 mg trenbolate acetate 16 mg estradiol |
Steers fed in confinement for slaughter; not for use in breeding herd replacements or dairy animals; not for use in veal calves |
Increase rate of weight gain and improve feed efficiency |
|
COMPONENT T-H2 |
Elanco Animal Health |
200 mg trenbolate acetate |
Feedlot heifers; not for use in breeding herd replacements or dairy animals; not for use in veal calves |
Increase rate of weight gain and improve feed efficiency |
|
COMPONENT TE-2002 |
Elanco Animal Health |
200 mg trenbolate acetate 20 mg estradiol |
Steers and heifers fed in confinement for slaughter; not for use in breeding herd replacements or dairy animals; not for use in veal calves |
Increase rate of weight gain and improve feed efficiency |
1U.S. Food and Drug Administration approved as of April 2011.
2Available with 29 mg Tylan, tylosin tartrate, as a local antibacterial.
Situations Where Implant Use Is Not Appropriate
As a general rule, do not implant breeding cattle, including bull and replacement heifer calves. Implanting bulls can result in problems in reproductive organ development and sterility. Implanting does not improve growth rate or efficiency in bulls. While some implants are labeled for use in replacement heifers, heifers can develop adequately without implants. It is advisable to implant only heifers to be marketed as feeders or stockers.
Side effects from implant use may include bulling, vaginal and rectal prolapses, udder development, and raised tailheads. Side effects are rare, of little economic significance in most cases, and not a reason to avoid implant use. These situations are often the result of improper implanting technique. Crushed implants may contribute to these conditions.
Some marketing programs specify that no implants be used on cattle in order for cattle to qualify for the programs. For example, “natural” programs may include such implant restrictions. Know the specifics of the targeted marketing program before using implants.
Implant Handling and Administration
Always use best management practices, including Beef Quality Assurance-compliant practices for implant use in beef cattle. Start by reading label directions on specific implant products. Label directions include information on the age, weight, and/or sex of cattle for recommended use of specific implants. Some implants require refrigerated storage or protection from light. Others require cool, dry storage, and still others should be stored at room temperature without excessive heat or humidity. The needed storage conditions will be indicated on the label. Review label instructions before implant storage and use. Check the product expiration date, and use implants before expiration.
Make sure the appropriate implant applicator (often called an implant gun) is on hand for use with the specific implant chosen. Manufacturers make implant guns specifically designed for certain implants. Match implants to the correct implant guns to minimize implant defects. Load the implant gun according to label directions. Use only sharp needles in implant applicators. Dull or burred implant applicator needles increase the risk of tissue damage and infection at the implant site. Burrs on needles can also damage implants. Check periodically for clogged implant applicator needles. Wash clogged needles with water and then disinfectant, and allow to dry before reuse.
Effective animal restraint makes implant administration easier and more likely to be done properly. Catching cattle in a head gate just behind the ears is ideal when implanting. With horned cattle, nose tongs can provide additional animal restraint and handler safety. Once a calf is properly restrained, select an appropriate ear for implanting. Select the ear with fewer ear tags, tattoos, and ear notches. If ears are tagged during the same cattle working event, then administer tags before implants. Try to tag calves in the opposite ear from the implant site. When possible, choose the same ear to implant in all calves worked together. This helps in monitoring implants later.
Find the proper implant location on the ear. Proper implant placement is under the skin on the backside of the ear (Figure 1). Administer them in the middle third of the ear between the skin and cartilage. The needle insertion site should be a point toward the tip of the ear at least a needle’s length away from the intended deposition site. Never place an implant in the cartilage ribs of the ear and never closer to the head than the edge of the cartilage ring farthest from the head. If the implant site is contaminated with mud or manure, scrape the site with a dull serrated knife, and clean the site with disinfectant before implanting. Do not contaminate the site with dirty hands. For reimplantation, place the second implant parallel to but not in contact with the previous implant or in the unimplanted ear.
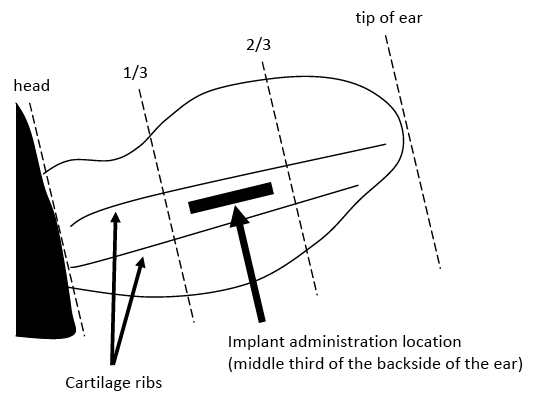
Grasp the ear to be implanted with one hand, and position the loaded implant applicator parallel to the backside of the ear. With the tip of the needle, prick and lift the skin to completely insert the needle under the skin, avoiding major blood vessels. The needle should form a canal between the skin and cartilage for deposit of the implant. Be careful to avoid gouging or piercing the cartilage. Needle resistance may indicate that the needle is gouging the cartilage. Once the needle is completely inserted, back it up slightly (about one-eighth to one-fourth of an inch). Some implant guns have retractable needles that eliminate the need for pulling the needle back slightly. Depress the trigger of the implant gun, and withdraw the needle slowly and steadily. Implant pellets should be deposited in a row. Gently palpate the ear to make sure the implant was properly inserted. Pellets should not be bunched or crushed, and the full dosage of implant pellets should have been deposited.
Improper implant administration can make the implant less effective or ineffective. Never sacrifice proper implant administration and sanitation for speed. Make sure everyone administering implants is trained in acceptable implant handling and administration techniques. Select the most conscientious crew member to administer implants. Periodically check implant technicians to make sure they are using good implanting technique. There are several common potential causes for implant failure. Many, if not all, of these causes are preventable.
Potential causes for growth-promoting implant failure in beef cattle:
- Missing implant (through the ear)
- Partial implant (due to implant gun failure or poor technique)
- Crushed or bunched implant pellets
- Improper implant site (in the cartilage)
- Abscess (due to poor sanitation or implanting technique)
- Inadequate implant storage (moisture, refrigeration)
- Inappropriate implant timing or target animal
Abscesses often result from infected implant sites. Abscesses may wall off the implant, preventing absorption, or push implant pellets out of the implant site. Adequate sanitation during implanting can help prevent abscess development. Thoroughly disinfect implant needles between animals. Wipe implant applicator needles with cotton or gauze moistened with a suitable disinfectant. Consider fly control measures when implanting during fly season.
Keep thorough and accurate implanting records. Record the date of administration, product administered, location of administration, and unique animal identification. An animal health processing map may be useful for these records. Keep the records, and inform buyers or future managers of past implant management. This helps prevent poor implanting decisions in later production phases.
Beef Safety
The FDA requires no withdrawal period before harvest of implanted cattle. Beef from implanted cattle has very low levels of estrogenic activity compared to many other common foods. Many commonly consumed foods, including vegetables and vegetable products, have much higher estrogenic activity than beef. In addition, the potential amount of estrogen consumed in beef is extremely low in comparison to that produced daily in the human body.
|
Food |
Estrogenic activity, nanograms per pound of food |
|---|---|
|
Soybean oil |
908,000 |
|
Eggs |
15,890 |
|
Cabbage |
10,896 |
|
Ice cream |
2,724 |
|
Peas |
1,816 |
|
Beef from a pregnant cow |
636 |
|
Milk |
59 |
|
Beef from implanted cattle |
10 |
|
Beef from nonimplanted cattle |
7 |
Adapted from Preston, 1997.
|
Item |
Estrogen produced, nanograms per day |
|---|---|
|
Pregnant woman |
90,000,000 |
|
Nonpregnant woman |
5,000,000 |
|
Adult man |
100,000 |
|
Prepubertal children |
40,000 |
|
3 ounces beef from implanted cow |
1.9 |
Adapted from Preston, 1997.
For more information on growth-promoting implant use in beef cattle production, contact your local MSU Extension office.
References
Duckett, S. K. & J. G. Andrae. 2001. Implant strategies in an integrated beef production system. Journal of Animal Science 79(E. Suppl.):E110–E117.
FDA. 2011. Food and Drug Administration approved animal drug products database. Available at: http://www.fda.gov/AnimalVeterinary/Products/ApprovedAnimalDrugProducts/default.htm.
Preston, R. 1997. Implants for suckling steer and heifer calves and potential replacement heifers. Symposium: Impact of implants on performance and carcass value of beef cattle. Oklahoma Agri. Exp. Station. P-957.
U.S. Department of Agriculture. 2009. National Animal Health Monitoring System BEEF 2007–08. Washington, D.C.
Reviewed by Brandi Karisch, PhD, Associate Extension/Research Professor, Animal and Dairy Science. Written by Jane A. Parish, PhD, Professor and Head, North Mississippi Research and Extension Center; and Justin D. Rhinehart, PhD, former Assistant Extension Professor, Animal and Dairy Science.
The Mississippi State University Extension Service is working to ensure all web content is accessible to all users. If you need assistance accessing any of our content, please email the webteam or call 662-325-2262.





