Adjusting Soil pH in Mississippi Landscapes
Soil pH indicates whether the soil is acidic, neutral, or alkaline. A soil pH of 7.0 is neutral; below 7.0 is acidic, and above 7.0 is alkaline. Most soils in Mississippi are acidic, but soil pH levels range from 4.0 to 8.0.
One alkaline area of the state extends from Boonville to Macon, which is referred to as the Northeast Prairie or Black Belt. Another extends from Jackson southeast to Wayne County and is known as the Central Prairie. Some of the Delta soils are alkaline, but most of the soils in the state are acidic.
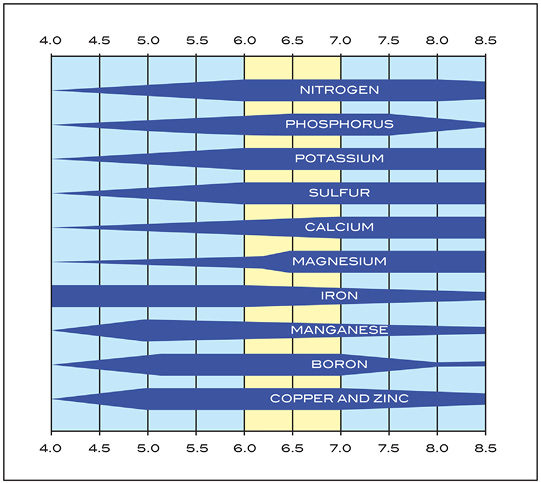
Soil pH influences the availability of plant nutrients. With the exception of hydrogen, carbon, and oxygen, the nutrients for growth are supplied to plants through the soil. Macro- and micronutrient availability for plant growth is strongly influenced by soil pH. Macronutrients, such as nitrogen, phosphorus, potassium, calcium, magnesium, and sulfur, are most available between 6.5 and 8.0 soil pH. Micronutrients are generally most available in acidic soils. A soil pH of 6.5 seems to be ideal for nutrient availability. See Figure 1 for the availability of specific nutrients across the pH scale.
It is important to know the soil pH and nutrient requirements of your ornamental plants to ensure plant growth and success. Most ornamental plants grow well in a pH range of 5.8 to 7.0. Exceptions are strongly acid-loving azaleas, gardenias, and camellias. Plants that are grown outside of their optimal pH range may develop nutrient deficiencies and grow poorly. For help determining which nutrients are lacking in ornamentals, contact your local Extension office. In general, it is best to select plants that are adapted to your soil pH, rather than attempting to alter the soil. For information on the pH requirements of common landscape plants, contact your local county Extension office.
Raising Soil pH
Lime is added to make soils less acidic. The amount needed to adjust the soil pH to the desired point varies based on many factors. These include soil texture, organic matter, and many soil chemical properties.
Choosing a Lime Type
Lime is available in several different forms. It can be purchased as a powder (ground or pulverized lime) or in a granular or pelletized form. The finer the lime is ground, the faster it will change the soil pH. The granular and pelletized forms have the benefit of being easier to apply through a fertilizer spreader. The pulverized form is much more likely to be very dusty and clog the equipment.
Lime also comes in several chemical forms. It can be calcium carbonate, dolomite, or hydrated lime. Dolomite and calcium carbonate are very similar in the effect they have on soil pH. Dolomite has the added benefit of supplying magnesium, a nutrient that may also be lacking in lower pH soils. Hydrated lime must be used with caution. It has a greater ability to neutralize acid in soils but also a greater potential to damage desirable plants.
Wood ash is generally less concentrated than lime but can raise the pH of a soil with repeated applications. Take care to keep wood ash away from plant roots and germinating seeds to avoid damaging desirable plants. Avoid applying large amounts of wood ash to avoid overliming and excessively high pH. Check the soil pH annually if wood ash is used as a liming material.
Applying Lime
To determine how much lime you need to raise the soil pH, you should take a soil sample and follow the soil report recommendations. Information on soil testing is available at your county Extension office or online at http://extension.msstate.edu/content/soil-testing. If you know your soil pH and a soil test with a lime recommendation is unavailable, you may want to apply 5 pounds of lime per 100 square feet. This rate may change the soil pH by 0.5 to 1.0 unit.
Lime is most effective if it is mixed into the soil thoroughly. This can be done easily before planting. Incorporating lime into the soil may not be possible in existing turf or around established plants. In these cases, apply the lime to the soil surface and water it in.
The best results are achieved by applying lime 2–3 months before planting. This allows the lime time to properly neutralize the soil acidity. A good practice is to take soil samples in the fall to determine how much lime needs to be added for the following year.
Lowering Soil pH
Lowering soil pH is much more difficult than raising pH. In fact, it is nearly impossible to permanently decrease the pH of soils formed from high-calcium materials, like those found in Mississippi. Problem soils can be created where concrete or cement was buried around a new house, building, or road median. Again, it is best to select plant material that is adapted to the existing soil pH rather than trying to correct the soil.
Sulfur can be added to soils to temporarily lower pH. Soil bacteria break the sulfur down into sulfuric acid, which helps neutralize soil alkalinity. This process takes time and depends on how finely the sulfur was ground, the soil moisture, and the population of soil microbes. Sulfur is most effective if it can be incorporated into the soil. If that is not possible, it should be watered in. The soil pH will begin to rise again when the microbes have used up all of the sulfur that was applied. This means multiple applications must be made to maintain the pH at the desired level.
If you use sulfur, be sure to monitor plants for any damaging effect. It is important not to add more than 2 pounds of sulfur per 100 square feet per year. It is best to split the applications, using one-half pound per 100 square feet every 3 months. Adding sulfur too frequently or at an excessive rate can damage desirable plants. See Table 1 for application estimates.
Aluminum sulfate is another soil amendment that is frequently used to lower soil pH. It is much faster-acting than elemental sulfur. It is readily available at most garden centers but is more expensive than sulfur. It requires about six times the amount of aluminum sulfate to provide the same effect as sulfur. It is generally best to mix the aluminum sulfate with water before applying it. Because excessive amounts of aluminum are toxic to plants, aluminum sulfate should never be applied at a rate greater than 5 pounds per 100 square feet. See Table 1 for application estimates.
Avoid getting either product on desirable plant leaves, if possible, and wash leaves off immediately after application to avoid damage. Take great care not to overapply sulfur or aluminum sulfate.
Amending your soil with organic materials such as compost, peat, or pine bark will affect the soil pH and has the added benefit of improving soil infiltration, fertility, and organic matter. Other fertilizers have an effect on soil pH, as well. See Table 2 for more information.
About 6 weeks after adjusting the soil pH, take another soil sample to see if further adjustment is needed to reach the desired pH.
|
Existing pH |
Target pH: 6.5 |
Target pH: 6 |
Target pH: 5.5 |
Target pH: 5 |
Target pH: 4.5 |
|---|---|---|---|---|---|
|
8 |
2 |
*2 2/3 |
*31/3 |
*4 |
*42/3 |
|
7.5 |
11/3 |
2 |
*22/3 |
*31/3 |
*4 |
|
7 |
2/3 |
11/3 |
2 |
*22/3 |
*31/3 |
|
6.5 |
0 |
2/3 |
11/3 |
2 |
*22/3 |
|
6 |
0 |
0 |
2/3 |
11/3 |
2 |
|
Existing pH |
Target pH: 6.5 |
Target pH: 6 |
Target pH: 5.5 |
Target pH: 5 |
Target pH: 4.5 |
|---|---|---|---|---|---|
|
8 |
*3 |
*4 |
*5 |
*6 |
*7 |
|
7.5 |
2 |
*3 |
*4 |
*5 |
*6 |
|
7 |
1 |
2 |
*3 |
*4 |
*5 |
|
6.5 |
0 |
1 |
2 |
*3 |
*4 |
|
6 |
0 |
0 |
1 |
2 |
*3 |
|
Existing pH |
Target pH: 6.5 |
Target pH: 6 |
Target pH: 5.5 |
Target pH: 5 |
Target pH: 4.5 |
|---|---|---|---|---|---|
|
8 |
*41/2 |
*6 |
*71/2 |
*9 |
*101/2 |
|
7.5 |
*3 |
*41/2 |
*6 |
*71/2 |
*9 |
|
7 |
11/2 |
*3 |
*41/2 |
*6 |
*71/2 |
|
6.5 |
0 |
11/2 |
*3 |
*41/2 |
*6 |
|
6 |
0 |
0 |
11/2 |
*3 |
*41/2 |
*Do not apply more than ½ lb per 100 ft2 every 3 months (2 lb per 100 ft2 of sulfur per year). If more is needed, split the applications and apply over multiple years.
|
Existing pH |
Target pH: 6.5 |
Target pH: 6 |
Target pH: 5.5 |
Target pH: 5 |
Target pH: 4.5 |
|---|---|---|---|---|---|
|
8 |
*12 |
*16 |
*20 |
*24 |
*28 |
|
7.5 |
*8 |
*12 |
*16 |
*20 |
*24 |
|
7 |
4 |
*8 |
*12 |
*16 |
*20 |
|
6.5 |
0 |
4 |
*8 |
*12 |
*16 |
|
6 |
0 |
0 |
4 |
*8 |
*12 |
|
Existing pH |
Target pH: 6.5 |
Target pH: 6 |
Target pH: 5.5 |
Target pH: 5 |
Target pH: 4.5 |
|---|---|---|---|---|---|
|
8 |
*18 |
*24 |
*30 |
*36 |
*42 |
|
7.5 |
*12 |
*18 |
*24 |
*30 |
*36 |
|
7 |
*6 |
*12 |
*18 |
*24 |
*30 |
|
6.5 |
0 |
*6 |
*12 |
*18 |
*24 |
|
6 |
0 |
0 |
*6 |
*12 |
*18 |
|
Existing pH |
Target pH: 6.5 |
Target pH: 6 |
Target pH: 5.5 |
Target pH: 5 |
Target pH: 4.5 |
|---|---|---|---|---|---|
|
8 |
*27 |
*36 |
*45 |
*54 |
*63 |
|
7.5 |
*18 |
*27 |
*36 |
*45 |
*54 |
|
7 |
*9 |
*18 |
*27 |
*36 |
*45 |
|
6.5 |
0 |
*9 |
*18 |
*27 |
*36 |
|
6 |
0 |
0 |
*9 |
*18 |
*27 |
*Do not apply more than 5 lb per 100 ft2 of aluminum sulfate in a single application. If more is needed, split the applications.
Caution: This information is for educational and preliminary planning purposes only. Use these tables only as a guide. The user assumes the risk of using or otherwise relying on the output of the table. The Mississippi State University Extension Service does not warranty the functionality of the table and concedes that errors can or will be discovered or corrected. MSU Extension does not warranty the accuracy or completeness of any output from the table. The table, its use, and its output are provided “as is” and without any expressed or implied warranty, including merchantability or fitness for a particular purpose. The Mississippi State University Extension Service is not bound by any table output and is not responsible for use or reliance on any such output.
|
Material |
Analysis N-P-K |
Effect on pH |
Speed of reaction |
|---|---|---|---|
|
ammonium sulfate |
20-0-0 |
very acid |
rapid |
|
sodium nitrate |
15-0-0 |
neutral |
rapid |
|
calcium nitrate |
15-0-0 |
neutral |
rapid |
|
potassium nitrate |
13-0-44 |
neutral |
rapid |
|
ammonium nitrate |
34-0-0 |
acid |
rapid |
|
urea |
45-0-0 |
acid |
rapid |
|
mono-ammonium phosphate |
11-48-0 |
acid |
rapid |
|
di-ammonium phosphate |
18-46-0 |
acid |
rapid |
|
triple superphosphate |
0-46-0 |
neutral |
medium |
|
superphosphate |
0-20-0 |
neutral |
medium |
|
muriate of potash (potassium chloride) |
0-0-60 |
neutral |
rapid |
|
sulfate of potash (potassium sulfate) |
0-0-50 |
neutral |
rapid |
|
limestone |
none |
basic |
slow |
|
hydrated lime |
none |
basic |
rapid |
|
gypsum (calcium sulfate) |
none |
neutral |
medium |
|
sulfur |
none |
acid |
slow |
|
epsom salts (magnesium sulfate) |
none |
neutral |
rapid |
|
aluminum sulfate |
none |
very acid |
rapid |
|
urea formaldehyde |
38-0-0 |
slightly acid |
slow |
|
dried blood |
12-0-0 |
acid |
medium |
|
steamed bone meal |
0-12-0 |
neutral |
slow |
|
cottonseed meal |
7-2-2 |
acid |
slow |
|
hardwood ashes |
0-1-5 |
basic |
medium |
|
linseed meal |
5-1-1 |
acid |
slow |
References
Pettry, D.E. 1977. “Soil Resource Areas of Mississippi.” MAFES Information Sheet 1278.
Shober, A., C. Wiese, G. Denny. 2011. “Soil pH and the Home Landscape or Garden.” UF-IFAS Extension Publication SL 256.
Williamson, J., M. Kluepfel, and B. Lippert. 2012. “Changing the pH of Your Soil.” Clemson Cooperative Extension Publication HGIC 1650.
The information given here is for educational purposes only. References to commercial products, trade names, or suppliers are made with the understanding that no endorsement is implied and that no discrimination against other products or suppliers is intended.
Publication 2831 (POD-04-22)
Revised by Keri Jones, PhD, Laboratory Coordinator, Plant and Soil Sciences; from an earlier version by Karl K. Crouse, PhD, Former Associate Extension Professor, and Geoffrey C. Denny, PhD, former Assistant Extension Professor, Plant and Soil Sciences.
The Mississippi State University Extension Service is working to ensure all web content is accessible to all users. If you need assistance accessing any of our content, please email the webteam or call 662-325-2262.


