Nematodes in Mississippi Soybeans: A Case Study Evaluating Sampling and Transportation Methods
Plant-parasitic nematodes are hidden threats to almost every crop grown for food and fiber. These tiny, worm-like invertebrates can be so small that they cannot be viewed without a microscope or hand lens. Being microscopic, nematodes frequently go overlooked as pests and are not properly managed.
A 2009 survey suggested that soybean yields decline 3.6 percent nationwide due to nematode infestations. In 2017, Mississippi’s 2.17 million acres of soybeans had an average yield of 53 bushels per acre (total production of 115 million bushels). Management options to prevent the 3.6 percent loss from nematodes would have generated an additional 4.14 million bushels and increased farm income by $38.51 million ($9.30 per bushel).
Many nematode species feed on soybeans, but three species are responsible for most yield losses. They are the soybean cyst nematode (Heterodera glycines), root-knot nematode (Meloidogyne spp.), and reniform nematode (Rotylenchulus reniformis). Nematode damage is often misdiagnosed as a disease or a nutrient deficiency, which is understandable. Foliar symptoms can be very similar, and soils are not routinely sampled for nematode infestations. Nematodes may cause wilting, root galls, overall plant stress, a reduction in nitrogen-fixing nodules, and stunting and yellowing of foliage (Figures 1 and 2).


Stunting, yellowing, and wilting are caused when the parasites feed so severely on plant roots that water and nutrient demands can no longer be supported. These symptoms can develop even when nutrients are readily available in the soil. Root galls typically occur when the plant is being attacked by root-knot nematodes; the galling forms where female nematodes attach to the root, causing tissue calluses. Rhizobium nodule formation may be reduced when nematodes are present. These nodules transform atmospheric nitrogen into ammonia (NH3), which is available to living plants.
Nematode feeding sites are believed to prevent some rhizobia bacteria from attaching to plant roots, which then reduces total nitrogen available to a plant. Because producers rely on this “free” nitrogen to supply most nitrogen requirements, reduction in nodules can reduce soybean yield.
Rationale for Case Study
Producers regularly notice soybeans with yellow foliage or stunted growth in irregular patterns within a field. These areas are often blamed on low fertility or a disease pathogen. Frequently, laboratory analysis may determine the culprit to be one of several nematode species. Producers may waste money attempting to manage a disease that does not exist or applying fertilizer when soil nutrients are already at sufficient levels.
When sampling for nematodes, it is possible to receive a negative report that indicates zero or low populations even though they are present and causing economic loss. This incorrect analysis may be due to reasons such as sampling at the wrong time, poor sampling technique, or poor handling/delivery of samples. Experts believe proper sampling, handling, and shipping techniques are critical for accurate data and treatment recommendations. Most experts agree that sampling should be representative of the field and samples should be stored out of direct sunlight by placing them in a cool location as soon as possible. Accurate sampling methods that determine true nematode populations should help producers increase soybean yield and profitability.
Input from producers, along with observations by agricultural professionals in north Mississippi, led to the development of a case study focused on rising nematode issues in soybeans. The following objectives were completed in this study: 1) to compare nematode sampling technique accuracy of growers (untrained) versus Extension specialists (trained); and 2) to compare nematode populations from samples transported via U.S. Postal Service (USPS) ground transportation versus direct delivery by Extension specialists.
Proper sampling technique is critical for accurate results. Sampling too shallowly, during cold weather, or in wheel tracks (rather than the root zone) can misrepresent populations. Analysis of these samples may not capture the true magnitude of nematode populations.
The author of this publication conducted a blind study in cooperation with five producers who were unaware of his goals and objectives until sampling was completed. Producers were not given any information other than that a nematode sample was needed for a study. Each producer collected one nematode sample from within a 1-acre confinement area located on their operation. Once the producer had completed sampling, an Extension specialist collected a sample from the same confinement area. Samples were collected on Tuesday, August 22, 2017, from soybean fields the specialist believed to show foliar symptoms of nematode infestation.
Delivery of samples via USPS is common due to the extra time and expense involved in personally delivering samples to a laboratory. Most professionals recommend immediate, direct delivery over shipping because laboratory analysis only counts living nematodes. If samples dry out during shipping and nematodes die, laboratory analysis will not accurately indicate the level of infestation that is present in the field.
For this part of the study, the Extension specialist sample was divided into three subsamples: 1) Sample 0, sample stored in an ice cooler and driven directly to the lab on Tuesday (zero hours in hot vehicle); 2) Sample 30, stored in a hot vehicle for 30 hours before USPS shipment on Wednesday at 4 p.m.; and 3) Sample 78, stored in a hot vehicle for 78 hours before USPS shipment on Friday at 4 p.m.
It was expected that samples stored for 30 or 78 hours inside a hot vehicle would have significant nematode mortality, resulting in lower populations than samples directly delivered to the laboratory.
Results
Laboratory analysis showed all samples had below-threshold populations for reniform, lesion, spiral, lance, stunt, and sheath nematode species. Therefore, these species will not be discussed. Root-knot nematode (RKN) populations exceeded threshold in four of five fields, and soybean cyst nematode (SCN) populations exceeded threshold in five of five fields.
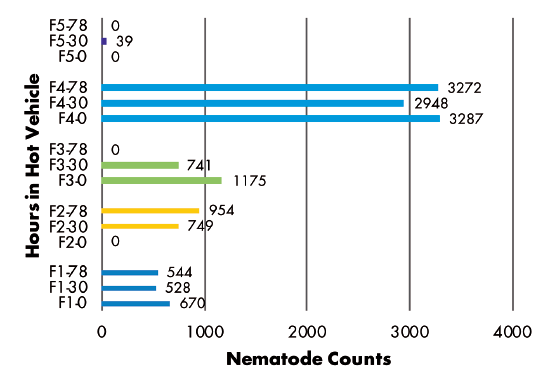
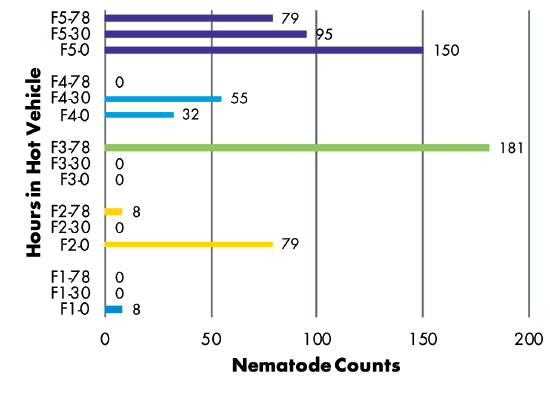
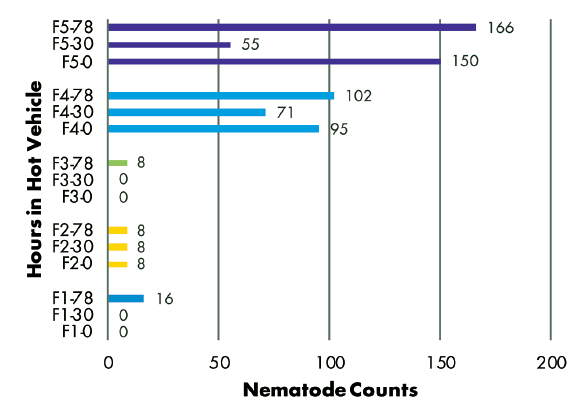
Delivery Method: Analysis of RKN populations showed no correlation with the number of hours samples were held in a hot vehicle between sampling and delivery to the laboratory. Samples from three fields had similar populations regardless of time held, while the population in one field decreased and the population in another field increased over time (Figure 3). SCN juvenile populations decreased over time in three of five fields, but increased over time in two fields (Figure 4). SCN cyst stage populations increased in four fields but were unchanged in one field (Figure 5).
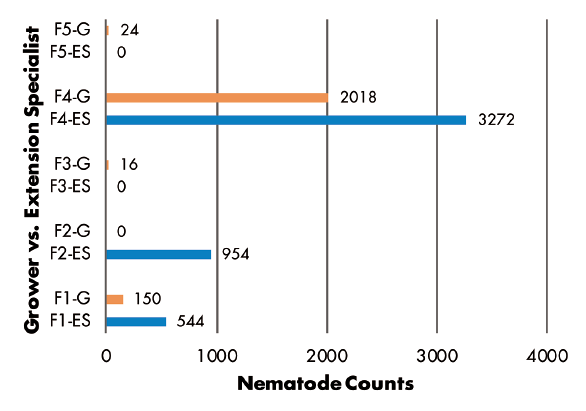
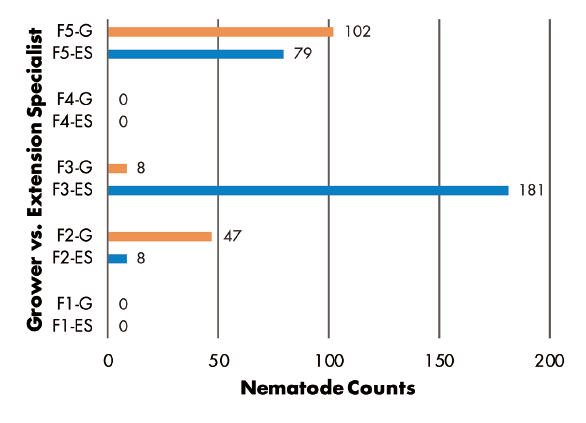
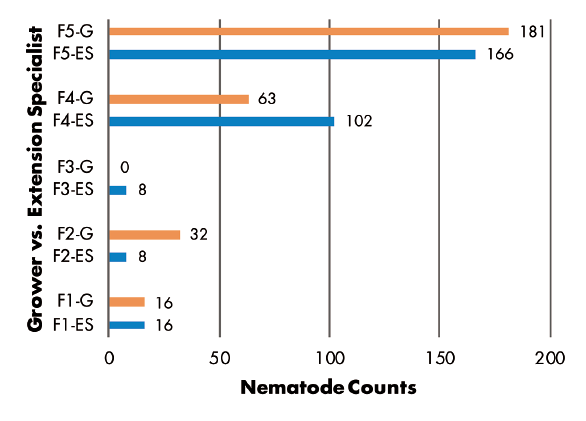
Grower vs. Extension Specialist: RKN populations were greater in samples collected by Extension specialist in three of five fields, but greater numbers were found in samples from two other fields where growers made the collections (Figure 6). SCN juvenile counts were greatest in grower-collected samples in two fields and in a specialist-collected sample in only one field. Two fields had no juveniles detected (Figure 7). SCN cyst stage counts were evenly split, as grower-collected and specialist-collected samples had the greatest populations in two fields each. The remaining field had identical counts for grower and specialist samples (Figure 8).
Figures 3–8 Legend
ES = Extension Specialist, G = Grower
F(1-5) indicates grower location (F3 = Field 3)
0, 30, or 78 indicate number of hours sample was held in hot vehicle (F2-30 = Field 2, held 30 hours in hot vehicle)
Conclusions
Nematode sampling results did not follow expected patterns for either study objective. Some fields showed greater populations from samples taken directly to the laboratory, while other fields had greater populations when samples were stored in hot, dry conditions for days. The higher populations from samples stored for 30 or 78 hours in a hot vehicle contradicts traditional recommendations for proper sample handling. For comparisons of grower sampling versus Extension specialist sampling, the specialist found greater populations of root-knot nematodes, while SCN populations were completely random.
Literature states that nematode populations can fluctuate widely within a field due to factors such as temperature, moisture, host plant, amount of time host plant has been available, and population dynamic changes due to adults dying and successive generations hatching. Generational hatching may explain why populations fluctuated so greatly between subsamples analyzed over time (hours held).
Numerous samples had zero or below-threshold populations when directly delivered, while an identical subsample held for hours resulted in above-threshold populations. It appears that nematode analysis can result in a false negative when nematodes are actually infesting the soil. If laboratory results are negative—yet a field shows nematode symptomology such as stunting, yellowing, wilt, or death—it may be justified to submit another sample to overcome this generational effect on population counts.
The case study further demonstrates the increasing geographical distribution of above-threshold levels of RKN and SCN nematodes across northeast Mississippi.
Publication 3244 (POD-06-24)
By Bill Burdine, PhD, Extension Specialist II, North Mississippi Research and Extension Center.
The Mississippi State University Extension Service is working to ensure all web content is accessible to all users. If you need assistance accessing any of our content, please email the webteam or call 662-325-2262.







