P4132
Prevention and Early Diagnosis of Mastitis in Dairy Goats
Mastitis is inflammation in the udder. Subclinical mastitis can average 5–30 percent annually within small ruminant herds, with gram-positive bacteria as its primary agent cause. Mastitis can reduce teat condition and milk yield and increase somatic cell count. Prevention techniques, early diagnosis of subclinical or clinical mastitis, and udder health management practices are the best ways to reduce and prevent mastitis cases in a dairy herd.
Anatomy of the Udder
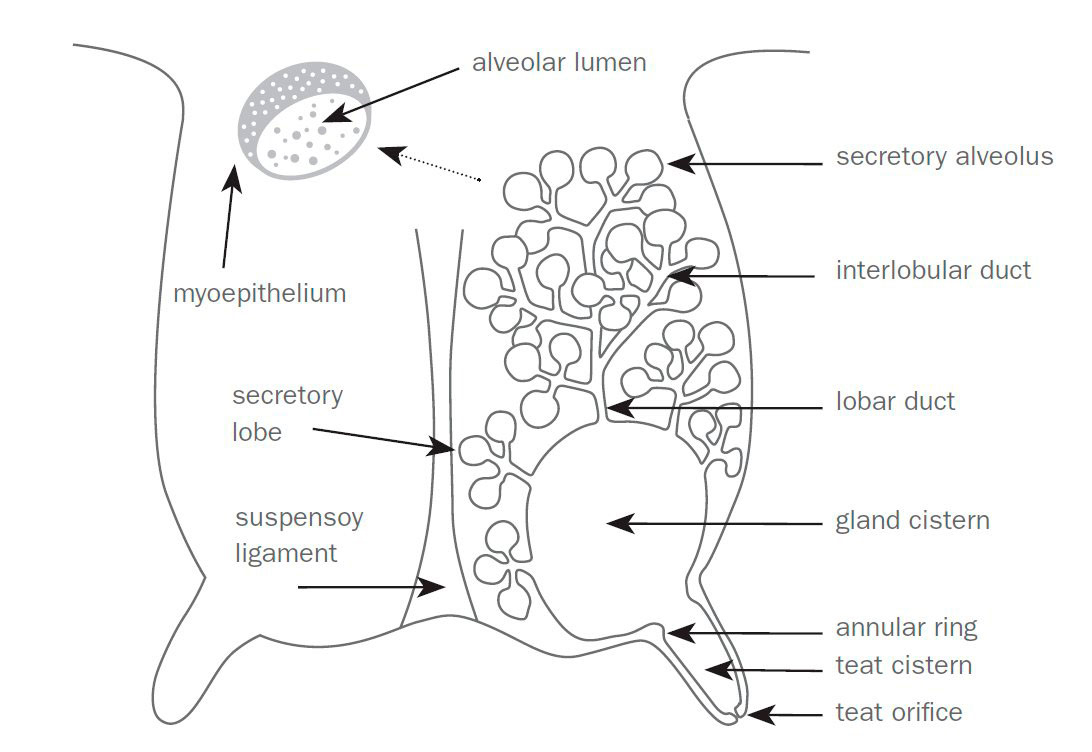
A dairy goat’s udder anatomy helps prevent infections, especially in the wild. Their teat and udder anatomy naturally blocks and prevents infections. For instance, the teat sphincter helps reduce microorganisms’ ability to enter the udder, while the keratin plugs (present during dry-off) act as a natural barrier in the teat sphincter. When using a milking machine, having a high-pressure system set to incorrect settings can damage the teat structure, making it less effective at preventing bacteria from entering the udder tissue.
Milk storage in the mammary gland and release varies with breed and lactation stage. Up to 70–90 percent of a goat’s total gland capacity is in the udder cisterns; in dairy sheep, 50–78 percent is in the cisterns. In comparison, cattle store most of their milk in their alveoli, accounting for up to 70 percent of total mammary capacity; the other 30 percent is stored in their cisterns. The high cisternal capacity in goats allows rapid milk letdown and faster machine milking but also makes the udder more vulnerable to ascending infections if machine vacuum settings are incorrect.
Early Diagnosis
California Mastitis Test (CMT)
The California mastitis test (CMT) is less accurate for goats mainly due to physiological differences in milk production. Unlike cows, healthy goats typically have a higher somatic cell count (SCC), which can cause false positives. The CMT is performed using the paddle provided in the kit and 2–3 milliliters of milk in each testing circle. It is important to mark which circle came from which udder half to monitor appropriately. Add an equal amount of reagent to the sample and swirl the mixture to determine if the udder half is infected.
The CMT works by detecting the lysis of somatic cells in the milk samples. The more somatic cells present, the more gel-like the mixture becomes. A high level of gel formation in the mixture can indicate a concerning somatic cell count. CMT scores of 1, 2, and 3 indicate positive results and should trigger laboratory testing. Take precautions when milking animals with these scores.
| CMT Score | Appearance |
|---|---|
| 0 (negative) | liquid; not gel-like at all |
| 1 | moderate gel formation |
| 2 | gel formation |
| 3 | strong gel formation; may stick to the paddle |
Somatic Cell Count (SCC)
Somatic cells within milk include lymphocytes, macrophages, eosinophils, neutrophils, cellular debris, and epithelial cells. High somatic cell counts (above 1 million cells per milliliter) indicate a likely case of mastitis. Normal SCC in goats varies widely (200,000 to over 1 million cells per milliliter) depending on breed, parity, and stage of lactation. A sudden increase in SCC for an individual animal is more indicative of infection than absolute values alone.
Somatic cell count (SCC) can be used to diagnose subclinical mastitis. Regularly test milk samples and record the SCC of each animal. Animals with an unusual increase in SCC could have subclinical mastitis. However, milk sample preservation and storage, estrous cycle, lactation stage, and sample age can affect the somatic cell count.
Techniques to Reduce Mastitis
Teat Dip
Pre- and post-milking dips (iodine- or chlorine-based) protect the udder by adding a chemical and physical barrier that prevents bacteria from entering and serves as a disinfectant. In addition, these dips can help maintain teat condition, preventing infections over time. For instance, post-dip creates a barrier for the teat end, reducing the opportunity for infection while giving the sphincter time to naturally close. Critical elements to consider when selecting a teat dip:
- It includes a broad-spectrum germicidal.
- The contact time works for your management plan.
- It is not irritating to the animal.
- It has no milk residue.
Environmental Factors
Environmental factors can increase an animal’s risk for mastitis. Wet and dirty environments can increase the risk of bacteria around the teats, increasing the animal’s risk of exposure. To reduce this risk, keep bedding dry, keep animals covered from the elements, properly clean milking machines, replace worn-out parts, and separate infected animals. Recommended bedding materials are straw, wood shavings, and sand. It is also important to have facilities with proper ventilation to reduce humidity and bacterial growth.
Check milking machine parts regularly and replace them as recommended by the manufacturer. To reduce the risk of transmission to other goats, separate animals with active infections and milk them on a different machine, by hand, or after all other animals have been milked. Washing hands before milking and using latex gloves can reduce the risk of bacteria passing to the udder from workers.
Dry-Off Therapy
Dry-off therapy involves using intramammary antibiotics during the dry period to prevent infections. Dry-off treatments vary; consult your veterinarian for the proper treatment protocol for your herd. Consider using alternative methods, such as selective dry-off therapy and teat sealants, especially considering current concerns about antibiotic resistance and withdrawal times.
Conclusion
Milking techniques and prevention programs are crucial in reducing an animal’s risk of mastitis. Using pre- and post-milking dips, keeping the animal’s environment clean, and monitoring udder health can significantly reduce the herd’s mastitis risk. Teat dip is one of the most effective tools in preventing new intramammary infections. These management practices can reduce the economic impact of mastitis (e.g., reduced milk yield, culling, treatment costs).
References
Balemi, A., Gumi, B., Amenu, K., Girma, S., Gebru, M., Tekle, M., Ríus, A. A., D’Souza, D. H., Agga, G. E., & Kerro Dego, O. (2021). Prevalence of mastitis and antibiotic resistance of bacterial isolates from CMT-positive milk samples obtained from dairy cows, camels, and goats in two pastoral districts in southern Ethiopia. Animals, 11(6), 1530.
Contreras, A., Luengo, C., Sánchez, A., & Corrales, J. C. (2003). The role of intramammary pathogens in dairy goats. Livestock Production Science, 79(2–3), 273–283.
Contreras, A., Sierra, D., Sánchez, A., Corrales, J. C., Marco, J. C., Paape, M. J., & Gonzalo, C. (2007). Mastitis in small ruminants. Small Ruminant Research, 68(1–2), 145–153.
Jimenez-Granado, R., Molina, A., Ziadi, C., Sanchez, M., Muñoz-Mejías, E., Demyda-Peyrás, S., & Menendez-Buxadera, A. (2022). Genetic parameters of somatic cell score in Florida goats using single and multiple traits models. Animals, 12(8), 1009.
Koop, G., van Werven, T., Schuiling, H. J., & Nielen, M. (2010). The effect of subclinical mastitis on milk yield in dairy goats. Journal of Dairy Science, 93(12), 5809–5817.
Manning, A., Vasileiou, N., & Crilly, J. (2021a). Control of mastitis in dairy sheep and goats. Livestock, 26(3), 161–168.
Marshall, R. T., Edmondson, J. E., & Steevens, B. (1993, October 1). Using the California mastitis test. University of Missouri Extension.
Menzies, P. (2021). Udder health for dairy goats. Veterinary Clinics of North America: Food Animal Practice, 37(1), 149–174.
Nuraini, D. M., Andityas, M., Sukon, P., & Phuektes, P. (2023). Prevalence of mastitis in dairy animals in Indonesia: A systematic review and meta-analysis. Veterinary World, 1380–1389.
Perrin, G. G., Mallereau, M. P., Lenfant, D., & Baudry, C. (1997). Relationships between California mastitis test (CMT) and somatic cell counts in dairy goats (PDF). Small Ruminant Research, 26(1–2), 167–170.
Sanz Franco, M. Á. (2021, May 7). Sheep and goat udders: Understanding the basics of anatomy and physiology. Small Ruminants.
Silanikove, N., Leitner, G., Merin, U., & Prosser, C. G. (2010). Recent advances in exploiting goat’s milk: Quality, safety, and production aspects. Small Ruminant Research, 89(2–3), 110–124.
The information given here is for educational purposes only. References to commercial products, trade names, or suppliers are made with the understanding that no endorsement is implied and that no discrimination against other products or suppliers is intended.
Publication 4132 (POD-10-25)
By Avery Permenter, Master’s Student, Leyla Ríos de Álvarez, PhD, Assistant Extension/Research Professor and Small Ruminant Specialist, and Jessica Halfen, PhD, Assistant Extension/Research Professor, Animal and Dairy Sciences. Reviewed by Santiago Cornejo, PhD, Assistant Professor, Animal and Dairy Sciences, and Carlos Alvarado, PhD, Dairy Product Specialist, Langston University.
The Mississippi State University Extension Service is working to ensure all web content is accessible to all users. If you need assistance accessing any of our content, please email the webteam or call 662-325-2262.
Authors
-
 Assistant Professor
Assistant Professor- Animal & Dairy Science
-
 Assistant Professor
Assistant Professor- Animal & Dairy Science
