P4129
Nematode Sampling for Crops
What Are Nematodes and Why Sample for Them?
Nematodes are worm-like organisms that inhabit a wide range of environments. While most nematodes live in water and soil as beneficial organisms, some parasitic nematodes can be harmful to humans, animals, and plants. Plant parasitic nematodes are microscopic, making them difficult to detect in soil or plant tissue. They are slender and typically measure between 0.2 and 4.0 millimeters in length and around 30 micrometers in diameter.
Most plant-parasitic nematodes feed on roots, disrupting the absorption of water and nutrients and hindering their movement to the upper parts of the plant. Nematode damage symptoms (stunting, yellowing, wilting, root galls, darkened root areas, root rotting, etc.) are often mistaken for nutrient deficiencies or other plant stressors, such as drought, flooding, diseases, and herbicide damage.
All major crops are affected by at least one species of plant-parasitic nematode, and they can damage both the quality and quantity of crop yields. Some nematode species can reduce yields by as much as 80 percent. These nematodes threaten many crops commonly grown in Mississippi, including cotton, soybeans, sweetpotatoes, corn, tomatoes, and other vegetables. In Mississippi, the most economically damaging nematodes (Figure 1) are reniform nematodes (Rotylenchulus reniformis), several species of root-knot nematodes (Meloidogyne spp.), and soybean cyst nematodes (Heterodera glycines).
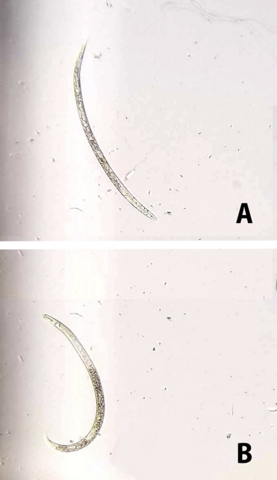
Another economically important species is the guava root-knot nematode (Meloidogyne enterolobii). While not currently found in Mississippi, it has become an emerging threat in several eastern states. If it becomes established in Mississippi, it could cause significant economic impacts across agronomic and ornamental crops (Figure 2).

Currently, effective management options include:
- use of resistant varieties.
- use of chemicals (nematicides).
- rotation to non-host crops.
Just as with weed control, overuse of any one control method can lead to resistance, so using an integrated pest management strategy is essential. The first step toward effective management of plant parasitic nematodes is to sample the soil for the presence and prevalence of nematodes before planting the crop. The sampling process is simple and inexpensive.
When to Sample
Nematode populations fluctuate with soil temperature and moisture, so timing is important while sampling. Sampling can be done:
- in fall (after crop harvest); best for determining overwintering populations.
- in spring (before planting); ideal for guiding planting decisions.
- during the growing season; from the root zone of the crop for any visible symptoms that may be present.
How Often to Sample
Sample fields every 3–5 years, depending on rotation, previous crop history, and observed issues. Fields with persistent yield problems may require more frequent monitoring.
How to Sample
- Use a soil map to determine different soil types and where they are distributed on the site to be sampled. Soil type can affect nematode populations, so handle different soil types as different samples. Soil maps are available online through the USDA Web Soil Survey (WSS). Contact your local Natural Resources Conservation Service (NRCS) or Extension office for more information.
- Collect a minimum of 15–20 soil cores (1 inch in diameter by 8 inches deep) per 10–20 acres of the same soil type. Correct depth is important for accurate measurements.
- Crops can show little to no obvious aboveground symptoms when infected by nematodes, making it tricky to select sampling areas. It is not appropriate to sample only visibly damaged areas. Soil testing is strongly recommended even if no obvious symptoms are noticed. Cores can be collected in one of three ways:
Zigzag pattern across the block (10–20 acres; Figure 3).
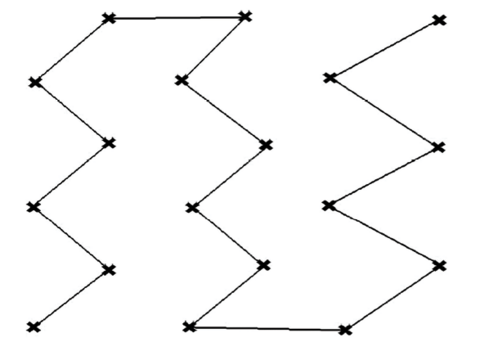
Figure 3. Example of zigzag sampling pattern across a 10- to 20-acre block (field). - Logical areas or management areas in the field. These are sections of the field that are managed differently or that naturally differ in characteristics like soil type, drainage, crop history, or productivity. Sample these areas separately because nematode populations can vary significantly based on how the area is used or maintained.
- High-risk areas (light, sandy soil; low-yielding areas; near field entrances; equipment or crop storage areas).
- Mix the cores well and place into a labeled nematode soil sample bag (wax-lined) or a ziplock bag.
- Keep the samples in a cool, dry place out of direct sunlight.
- Seal the sample bags and send them to the Extension Plant Diagnostic Lab with a completed Nematode Sample Submission Form (PDF).
- The analysis report will identify the nematode species detected in the sample along with their population densities. These figures are then evaluated against established threshold levels to determine whether management actions are necessary.
- The MSU Extension nematode soil test report will indicate the nematode concentration for various economically important species and whether the concentration of a particular nematode is at or near a level of concern.
Where to Submit Samples
After completing the Nematode Sample Submission Form, submit samples to the Extension Plant Diagnostic Lab.
Physical Address
Extension Plant Pathology Lab
Bost Extension Center
190 Bost North, Rooms 9 and 10
Mississippi State, MS 39762
Mailing Address (U.S. Postal Service)
Extension Plant Diagnostic Lab
Box 9612
Mississippi State, MS 39759
Shipping Address (FedEx/UPS)
Extension Plant Diagnostic Lab
405 Garrard Road East
Mail Stop 9612
Starkville, MS 39762
Nematode Testing Fees
Fees are subject to change. View current fees on the Extension Plant Diagnostic Lab web page.
Questions?
For more information on nematode identification, crop-specific management, or submitting large-scale samples, contact the MSU Extension Plant Diagnostic Lab or your local Extension agent.
Publication 4129 (POD-08-25)
By Varsha Singh, PhD, Postdoctoral Associate, Pontotoc Ridge-Flatwoods Branch Experiment Station; Lorin Harvey, PhD, Assistant Professor and Extension Sweetpotato Specialist, Pontotoc Ridge-Flatwoods Branch Experiment Station; Chang Liu, PhD, Assistant Professor, Agricultural Science and Plant Protection; Francis Kiemo, PhD, Postdoctoral Associate, Pontotoc Ridge-Flatwoods Branch Experiment Station; and Clarissa Balbalian, Diagnostic Lab Manager, Agricultural Science and Plant Protection.
The Mississippi State University Extension Service is working to ensure all web content is accessible to all users. If you need assistance accessing any of our content, please email the webteam or call 662-325-2262.
