P3722
Sustainable Parasite Control for Sheep and Goats

One of the main drawbacks in small ruminant production systems worldwide is gastrointestinal nematodes (GIN), or worms. The lack of options for controlling worms is mainly a result of increasing resistance these parasites have developed in the last decades. This has caused economic losses to farmers with grazing production systems.
Since 2001, GIN infection was the most prevalent disease, reported by 74 percent of the U.S. farmers surveyed in a National Animal Health Monitoring System study. According to the 2021 Census of Agriculture, U.S. producers have 5,170,000 sheep and 2,582,000 goats. In the last decades, these numbers have decreased annually. Studies are needed to determine if parasite resistance to commercial dewormers is a factor in this decrease.
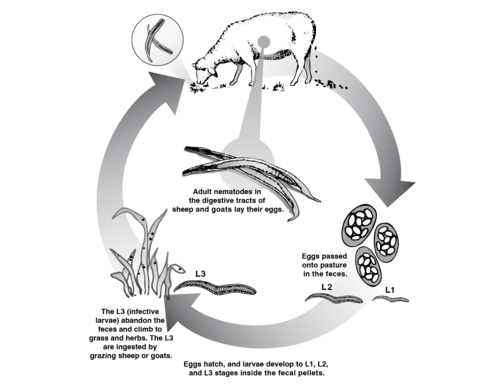
Gastrointestinal Worm Life Cycle
Most GINs that affect sheep and goats have a direct life cycle, meaning they pass from the environment directly to the final host, without using an intermediary one. Thus, the host (sheep or goat) has adult male and female worms in its gastrointestinal tract that mate, allowing female worms to begin excreting eggs.
Figure 1 shows that these eggs are excreted from the animal’s feces into the environment, where the soil humidity, temperature, and other weather conditions provide the perfect microenvironment for the eggs to hatch. During spring, summer, and autumn, environmental conditions favor egg hatching and stage 1, 2, and 3 larvae development. During winter, conditions are less adequate for all life stages.
When the eggs hatch, the L1 larvae are produced and develop into L2 larvae inside the feces, using bacteria as a food source. A second molt allows them to develop into L3 larvae (infective larvae) and migrate outside the feces, spreading into the pastures. When animals feed on forages, they ingest these L3s, and the cycle begins again.
The displacement of these L3s depends on the structure of the grassland, rain, soil moisture, and solar radiation. Therefore, L3 larvae are present in greater proportion in the lower strata of the grassland, and their survival and dissemination are favored by the presence of rains and the low intensity of sunlight.
Most Common Symptoms of Parasitized Animals
The GINs that most commonly affect sheep and goats are Haemonchus contortus (barberpole worm) and Teladorsagia circumcincta (brown stomach worm), both found in the final stomach compartment or abomasum, and Trichostrongylus colubriformis (bankrupt worm or black scour worm), found in the small intestine.
Common signs of gastrointestinal parasitism in both sheep and goats are weakness, loss of weight and body condition, bristly hair, “bottle jaw,” anemia, and diarrhea. There are specific clinical signs for the different parasitic species. In the case of T. colubriformis, signs can be confused with bad nutrition, starting with reduced intake, low growth rate, and soft feces; as the infestation increases, diarrhea becomes dark, and severe weight loss and even death can occur. With T. circumcincta, signs are similar, including decreased intake and loss of body weight; diarrhea is not always observed. Severe anemia is the main sign of H. contortus; after 2 weeks of infection, an average of 5,000 worms per sheep can consume approximately 250 milliliters of blood each day, resulting in a significant reduction in red blood cells.
The animals most affected by GIN will be those that have the largest worm burdens and at the same time face malnutrition in terms of quantity and quality. This could be more common in weaned animals and ewes around parturition and lactation.
Types of Dewormers
Pharmaceutical laboratories have three families of dewormers:
- Benzimidazole: fenbendazole (Safeguard) and albendazole (Valbazen)
- Levamisole: levamisole hydrochloride (Prohibit and Levamed), and morantel tartrate (Rumatel)
- Macrolytic lactones: ivermectin (Ivomec) and moxidectin (Cydectin)
In the United States, only fenbendazole, albendazole, and morantel tartrate have been approved by the FDA for use in goats. The other dewormers can be prescribed only by a veterinarian for “extra-label” use. For sheep, FDA-approved dewormers are albendazole, ivermectin, moxidectin, and levamisole. The American Consortium for Small Ruminant Parasite Control (ACSRPC) recommends combination treatments for clinically parasitized animals, using the most potent drug from each group, at the full dose, orally, one after the other, without mixing them, emphasizing that such treatment scheme must be targeted to animals with clinical evidence of parasites. However, farmers need to establish a veterinary-client-patient relationship to use these “extra-label” drugs. To control liver fluke, the only approved drug for goats is albendazole. Check the ACSRPC website for updates on these recommendations.
Sustainable Practices for Parasite Control
Approximately 60 years ago, the first commercial veterinary dewormer, called Thiabendazole, was produced. It took only 3 years for the first case of parasite resistance to be reported. Parasite resistance is a worldwide problem and, therefore, scientists around the world are looking for alternatives.
Sustainable parasite control usually refers to using alternatives that prevent resistance and minimize disease and production losses. Integrated parasite control combines traditional control with alternatives that do not depend exclusively on commercial dewormers. Visual indicators and other control measures are used to space chemical deworming and reduce resistance to commercial chemical compounds. The goal is to maintain refugia, which is a part of the parasite population on the farm that is maintained with no exposure to commercial dewormers, so it does not become resistant. Refugia is achieved by deworming only those animals that really need treatment (Figure 2).
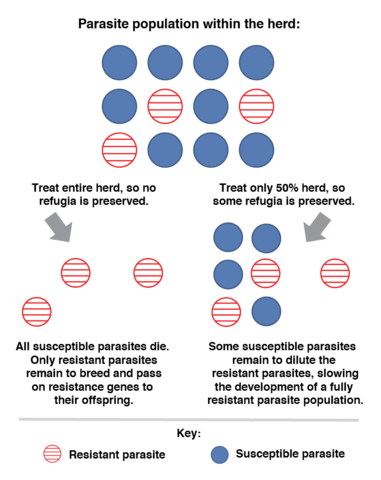
Estimating the Parasitic Burden of Animals
Counting worm eggs in an animal’s feces (fecal egg count, or FEC) is used to indirectly estimate the parasitic population. This estimation is made using the McMaster technique, which mixes feces (2 grams taken directly from the animal’s rectal ampoule) with a saturated solution of sugar or salt (28 cc of what is called flotation solution). This mixture is placed into a McMaster slide, where the eggs from GIN will float and can be counted. Some other uses of FEC are to determine drug resistance, identify resistant animals, and monitor pasture contamination.
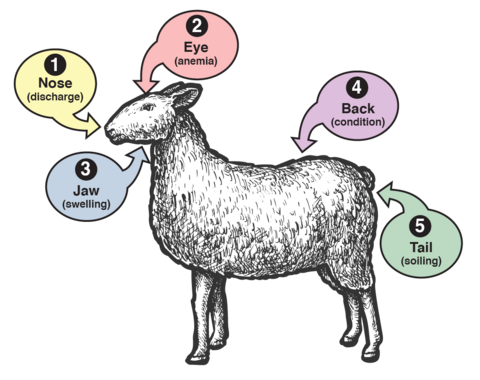
Indicators Suggesting Parasitism
Figure 3 shows the Five-Point Check, which summarizes other indicators suggesting parasitism in sheep and goats. These indicators, listed below, are important when deciding whether to deworm an animal in targeted selective treatment (TST).
- Nose (nasal discharge): Even when it is not an indicator of nematode infection, the clear or purulent nasal discharge can be caused by the nasal larval stages of the Oestrus ovis fly. In addition to affecting the welfare of the animals, this infection can lead to immunosuppression and bacterial infections.
- Eyes (mucous membranes of the lower eyelid): Trained individuals can examine the eyes for signs of anemia. Developed in South Africa in 1990, the FAMACHA chart is used as an indirect measure of anemia, which suggests high worm burdens of the hematophagous parasite H. contortus. For more information, see the ACSRPC website.
- Jaw (presence of “bottle jaw”): This swelling is a typical indicator of low levels of protein in the blood that could be associated with worm infections.
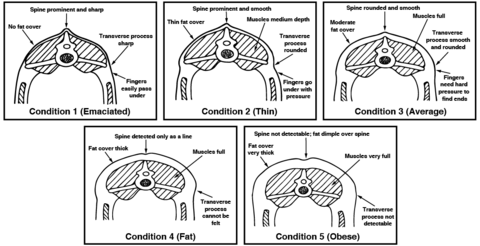
- Back (body condition score, or BCS): Body condition scoring is determined through observation and palpation of the back and sides of the animal. This assessment typically uses a scale from 1 to 5 (Figure 4). A poor BCS can be associated with high worm burden.
- Tail (presence of diarrhea, or dag): This scale measures the level of feces or diarrhea that accumulates on the rear end of animals and could be a sign of high worm infection.
Body condition score and dag score are subjective. For consistency in scoring, the same person should evaluate animals each time.
Alternative Practices for Parasite Control
Two kinds of practices can be included in a sustainable parasite control program: those that help control the parasite burden in the animal/host and those that help reduce infective L3 larvae from the pasture.
Control at the Animal Level
Selection/culling: Select the most productive and parasite-resistant animals to keep in the herd, and cull those that require constant deworming or that permanently present high parasitic loads. Consider culling older animals, which develop teeth problems that affect their intake and compromise their immunity, making them more susceptible to worms. FEC is the most accurate way to select for parasite resistance, and it is suggested that only rams/bucks with proven parasite resistance (EBVs, ram/buck tests) be used for breeding.
Parasite-resistant breeds: Some sheep and goat breeds tend to be more tolerant to parasitism and, despite their parasite load, grow and reproduce properly. For example, St. Croix was found to have a higher resistance to parasitism than other hair sheep breeds.
Selective deworming or targeted selective treatment (TST): Use indicators to maintain refugia, deworming only those animals that require it. Some indicators that can be used for TST or selective deworming are FAMACHA score (4 and 5), BCS score (lower than 3), weight loss, and diarrhea. Use any of these indicators or a combination of indicators.
Strategic supplementation: The relationship between adequate nutrition and an animal’s level of immunity to diseases is well known. Animals must receive a proper diet, especially during demanding stages such as the growth, reproduction, and milk production stages.
Alternative treatments: The American Consortium for Small Ruminant Parasite Control recommends giving the smallest effective dose of copper oxide wire particles (COWP), which is 0.5–1.0 grams for lambs/kids and 1–2 grams for adults. The 2 grams could be used for adult goats and less for sheep. COWP should be used as a targeted selective treatment, using FEC or FAMACHA as indicators. To avoid copper toxicity in animals, especially sheep, animals can be treated with COWP only once in a grazing season. For more information, see the ACSRPC's page Copper Oxide Wire Particles: Keeping It Safe.
Control at the Grazing Level
Adequate paddock rotation/rest periods: Allow enough time between groups of animals to prevent the larvae of the first group of animals from infecting the next group. An adequate resting period varies according to the weather and time of year. The life span of L3s in the grass is approximately 28–40 days in tropical conditions and up to 6 or more months in temperate conditions.
Graze young/low-immunity animals in “clean” paddocks or pastures that have not been grazed previously by contaminated adult animals.
Avoid overgrazing: The life cycle of the GIN indicates that most L3s will be in the lowest part of the forages, near the soil and feces. When animals overgraze paddocks, they eat closer to the lower parts of the forages, where there is a greater possibility of L3 contamination. Adjust stocking rate to paddock size to have enough forage during the grazing period.
Use bioactive forages or plants containing secondary metabolites: The leaves of some plants contain metabolites (tannins, saponins, lectins, and others) that can have a dewormer effect on animals and decrease their parasitic loads. Farmers will need to include temperate or tropical bioactive forages in their paddocks.
Use multispecies paddocks: Animals can select an appropriate diet that allows them to better cover their nutritional requirements, and better-fed animals are more able to resist parasitism.
Use plants of different heights: This allows animals (mainly goats) to alternate grazing with browsing and avoid the most contaminated parts of the pasture.
Use mixed animal species grazing: Grazing sheep and goats with other species, such as cattle and horses, decreases the levels of infective larvae in grazing areas. After sheep or goats finish grazing, cattle or horses can act as “vacuum cleaners” of L3 larvae, lowering the chance of infection in small ruminants. Sheep and goats are top grazers that prefer the upper, immature parts of forages, while cattle and horses prefer the lower parts. In tick-infested areas, mixed grazing is not advised because ticks will also affect sheep and goats.
Alternative treatments: A new option is to use nematophagous fungi (Duddingtonia flagrans, BioWorma), a nematode-trapping or predatory fungi that traps and kills larvae in the feces, preventing reinfection of pastures and animals.
Summary
Parasite resistance leaves very few options for farmers who rely only on traditional deworming with commercial dewormers. To minimize the progress of parasite resistance in sheep and goat herds, more holistic and sustainable approaches are needed, reducing the effect of GIN on animals and the spread of larvae in grazing areas.
References
Abongwa, M., Martin, R., & Robertson, A. (2017). A brief review on the mode of action of antinematodal drugs. Acta Vet (Beogr), 67(2), 137–152.
American Consortium for Small Ruminant Parasite Control (ACSRPC). (August 22, 2019). History of FAMACHA: Interview with Dr. Faffa Malan, Landbou [Video]. YouTube.
Bath, G., & van Wyk, J. (2009). The Five Point Check for targeted selective treatment of internal parasites in small ruminants. Small Ruminant Research, 86, 6–13.
Besier, R. (2012). Refugia-based strategies for sustainable worm control: Factors affecting the acceptability to sheep and goat owners. Veterinary Parasitology, 186, 2–9.
Burke, J., & Miller, J. (2004). Relative resistance to gastrointestinal nematode parasites in Dorper, Katahdin, and St. Croix lambs under conditions encountered in the southeastern region of the United States. Small Ruminant Research, 54, 43–51.
Drudge, J., Szanto, J., Wyant, Z., & Elam, G. (1964). Field study on parasite control in sheep: Comparison of thiabendazole, ruelene, and phenothiazine (login required). American Journal of Veterinary Research, 25(108), 1512–1518.
Kahn, L., & Woodgate, R. (2012). Integrated parasite management: Products for adoption by the Australian sheep industry. Veterinary Parasitology, 186, 58–64.
Kaplan, R. (2014a). Dewormer chart for sheep. American Consortium for Small Ruminant Parasite Control (ACSRPC).
Kaplan, R. (2014b). Dewormer chart for goats. American Consortium for Small Ruminant Parasite Control (ACSRP).
Mallet, R. (n.d.). Responsible anthelmintic use and resistance. Bimectin.
RVC. (2021). McMaster egg counting technique: Principle. The RVC/FAO Guide to Veterinary Diagnostic Parasitology.
Sangster, N. C., Cowling, A., & Woodgate, R. G. (2018). Ten events that defined anthelmintic resistance research. Trends in Parasitology, 34(7), 553–563.
Silva, B., Amarante, M., Kadri, S., Carrijo-Mauad, J., & Amarante, A. (2008). Vertical migration of Haemonchus contortus third stage larvae on Brachiaria decumbens grass. Veterinary Parasitology, 158, 85–92.
Taylor, M., Coop, R., & Wall, R. (2007). Veterinary Parasitology (3rd ed.). Blackwell Publishing.
Thompson, J. M., & Meyer, H. (1994). Body condition scoring of sheep. EC 1433. Oregon State University Extension Service.
USDA. (2003). Sheep 2001 Part II: Reference of sheep health in the U.S., 2001. [PDF]
USDA. (2021). Sheep and goats. [PDF] National Agricultural Statistics Service (NASS), Agricultural Statistics Board, United States Department of Agriculture (USDA).
U.S. Food and Drug Administration Center for Veterinary Medicine. (n.d.). Antiparasitic resistance in cattle and small ruminants in the United States: How to detect it and what to do about it.
Vatta, A., Waller, P., Githiori, J., & Medley, G. (2012). Persistence of the efficacy of copper oxide wire particles against Haemonchus contortus in grazing South African goats. Veterinary Parasitology, 190, 159–166.
Whittier, W. D., Zajac, A., & Umberger, S. H. (2009). Control of internal parasites in sheep. Virginia Cooperative Extension, Virginia Tech, and Virginia State University. Publication 410-027.
The information given here is for educational purposes only. References to commercial products, trade names, or suppliers are made with the understanding that no endorsement is implied and that no discrimination against other products or suppliers is intended.
Publication 3722 (POD-02-25)
By Leyla Ríos de Álvarez, PhD, Assistant Extension/Research Professor and Small Ruminant Specialist, Animal and Dairy Sciences. Reviewed by Susan Schoenian, Sheep and Goat Specialist Emeritus, University of Maryland Extension; Juan Felipe de Jesús Torres Acosta, PhD, Lecturer and Researcher, Facultad de Medicina Veterinaria y Zootécnia, Universidad Autónoma de Yucatán, México; Dean Jousan, MSU Extension; and Rocky Lemus, MSU Extension.
The Mississippi State University Extension Service is working to ensure all web content is accessible to all users. If you need assistance accessing any of our content, please email the webteam or call 662-325-2262.
