The Plant Doctor: Take-All Disease of Turfgrasses

Take-All Disease
Most active time of year
Common during June to October, but the disease can be diagnosed at any time.
Weather
Temperatures in the 80s, often with daily rains.
Turf types affected
Mostly St. Augustinegrass, but also affects bermudagrass, zoysiagrass, and centipedegrass.
Quick symptoms
Spreading dead areas, often starting near sidewalks or driveways. The first aboveground symptoms are irregularly shaped patches of slightly yellow turf. The turf transitions from yellow to brown (dead) over a period of several weeks. If you grasp the above ground parts of the plants in your hand and pull upward, stolons or rhizomes will break off in the soil, leaving you holding some nodes with leaves and dead roots (see Figure 3).
Our current understanding of the disease and pathogen
The pathogen(s) that cause take-all root rot are classified as ectotrophic root-infecting (ERI) fungi. ERI fungi live on the outside of the underground parts of the turf plants. They form thick, dark, thread-like strands (hyphae) along the length of the roots, stolons, and rhizomes (Figure 1a). Some of them also attack food crops, such as rice and wheat, as well as turf.
Identifying pathogenic ERI fungi and limiting the diseases they cause has been an evolving story since the first turf disease caused by them was identified and named “Bermudagrass decline” in 1984. In 1991, the pathogen causing Bermudagrass decline was identified as Gaeumannomyces graminis var. graminis (Ggg). By the late 1990s, the Ggg fungus was associated with declining St. Augustinegrass, centipedegrass and zoysiagrass home lawns throughout the southeast. The disease in residential lawns was called “take-all.” By 2000, the disease became distressingly common in St. Augustinegrass. Meanwhile, the suspicion grew that (in golf courses, at least) multiple, similar-looking, pathogens were involved.
Research published in 2015 by Mississippi State University turf pathologists described multiple ERI pathogens that were involved in diseases of turf on golf greens. To date, five different organisms in three genera are routinely isolated from turfgrass samples with symptoms of take-all root rot (mostly on golf greens). These organisms are Gaeumannomyces graminis (Gg), Gaeumannomyces sp. (Gx), Gaeumannomyces graminicola (Ggram), Candidacolonium cynodontis (Cc), and Magnaportheopsis cynodontis (Mc).
While a few people claim to be able tell them apart with good samples and a microscope, most identifications use some form of a laboratory DNA test. These tests tend to be expensive, and there are corporate barriers to their use by plant disease diagnostic laboratories. Research by North Carolina State University showed disease severity and fungicide efficacy differed depending on which fungus was infecting the plant.
As of now, the take-all pathogens involved in residential lawns and the role they play in disease is unclear. Although research by North Carolina State University showed disease severity and fungicide efficacy differed depending on which fungus was infecting bermudagrass greens, differences in management for the different fungi are wholly unknown.
Additionally, telling the fungi apart usually requires some form of a laboratory DNA test. These tests are expensive and their result will not assist in management. Until research provides management practices for at least one of the fungi, this publication will provide practices that have worked for others or are inferred from other crops the pathogens infect. Diagnosis will indicate “take-all disease” rather than specific pathogens. As our knowledge grows, management recommendations will change.
Successful disease management requires understanding the fungus interaction with the plant. As long as the plant is healthy, the hyphae stay on the surface of the plant structure (Figure 1a) and does not significantly influence plant health.
When the plant is stressed, its defenses are weakened, and it can no longer keep the fungus out. The fungus forms specialized structures, (hyphopodia) to penetrate the plant (Figure 1b, c). Once inside, the fungus slowly consumes plant tissues. Affected tissue first turns yellow, then dark brown or black as decline and decay continue. Roots, crowns, stolons, and rhizomes may all be infected.
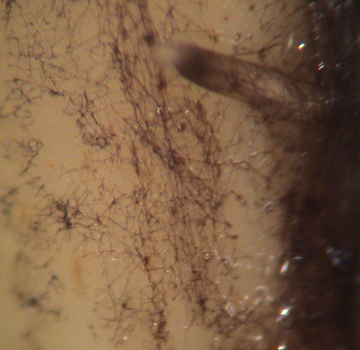


Figure 1a-1c. Views of St. Augustinegrass and bermudagrass stolons infected with an ERI fungus, probably Gaeumannomyces graminis. Figure 1a, top, features a washed St. Augustinegrass stolon with Ectotropic Root Infecting Fungi (ERI) (dark colored, thick) fungal hyphae growing in the typical long direction of the plant part. Figure 1b, middle, provides a size perspective of ERI infection. The infection is on a washed ultradwarf Bermudagrass (small) stolon and shows a sparse density of ERI fungal hyphae and the dots of hyphopodia to which many hyphae connect. Figure 1c, bottom, a close up of lobed hyphopodia on a washed stolon.
Figures 1b and c are courtesy of Clarissa Balbalian, Diagnostician/Laboratory manager, Mississippi Plant Diagnostic Lab.
The fungus may spread to other areas by contaminated equipment, sod, or rice stalks used for mulch. On rice, the fungus produces sexual spores, which may be spread by wind. You seldom see this stage in turfgrasses.
The fungus grows best at a soil pH higher than 6. Soil water levels near field capacity seem to stimulate fungal growth and development. Most damage seems to occur to the underground plant parts when water is abundant for sustained periods.
Optimal growth temperatures for all take-all fungi lies between 77 and 86° F. The temperature at which the growth rate accelerates (the inflection point), is around 68-70°F. Growth is greatly reduced between 86 and 95°F
Symptoms
Symptoms are most common in the summer and fall. Usually, temperatures are in the 80s, and daily rains are common. Irregularly shaped patches of off-color, slightly yellow to brilliant yellow turf are often the first visible symptoms after rainy periods (Figure 2). The color becomes more noticeable until the turf starts to brown and die. This happens faster in hot, dry weather than in cooler weather. The dead patches start small and enlarge greatly over a few years so that after three years, much of the lawn may be affected. The dying and dead patches correspond to the yellow patches seen earlier.
By the time you see the first yellowing symptoms (chlorosis) in the turf (Figure 2), the fungus has damaged much of the root system. Decayed roots are unable to supply adequate water and nutrients to the plant, leading to chlorosis. Roots affected by the disease appear dark, usually black, and rotted. As the disease progresses, stolons and rhizomes also rot, and turf fully affected by the disease does not hold onto the ground. If you grab a handful of the turf and pull on it, it comes out of the ground easily, with only a few blackened roots clinging to the nodes (Figure 3).


Figure 2a-2b. Yellow patches in St. Augustinegrass resulting from excessive rain which kept the soil near field capacity for protracted periods. ERI seem to grow quickly in these conditions. Examining the roots under the yellow patches will show they are beginning to decay (see Figure 3). The bright yellow color seen here occurs infrequently, typically it is a duller yellow.
These images are courtesy of Ms. Jessica L. Sibley, Wayne County Extension Agent.
Structures called lobed hyphopodia (Figure 1c) are diagnostic of take-all fungi infection. The lobed hyphopodia look like a cartoonist’s drawing of an amoeba, or a deeply scalloped circle—like a large cookie with bites taken from around the edges. You can see them with 20x or higher magnification (hand lens/microscopes) on washed stolons and rhizomes. They are most apparent on St. Augustinegrass stolons or leaf bases of zoysiagrass.
You can have the disease professionally diagnosed and receive a full report and recommendation from the Mississippi State University Extension Service Plant Diagnostic Laboratory (see M1230 Plant Disease and Nematode Diagnostic Services). Collect a 6-by-6 inch sample that includes the roots where the edge of the diseased area merges into healthy turf. Wrap the sample in dry newspaper, place it in a plastic bag, box it, and ship it with a completed plant disease sample submission form (F1139) to our diagnostic lab at the address on the form. Please see http://extension.msstate.edu/lab for the current form, fees, and instructions. Results are usually available within 3 to 7 days of receiving the sample and payment.
Management
Since the fungus apparently does not become pathogenic until the host plant is under stress, eliminating stress on the turf plants is an important part of turf recovery. You should do a complete survey of all cultural practices that cause stress and change those practices that can stress turf.
Common turf stress factors are mowing height, mowing frequency, soil pH, fertility, herbicides, watering, compaction, and thatch. Only brief summaries of these stress factors are provided here, but proper management practices for warm-season turfgrasses are outlined in Extension publication 1322 Establish and Manage Your Home Lawn.
The first step in managing the disease is to have a soil test done for each major section of your turf area or lawn. Take your soil samples from the top 4 inches because this is where most of the plant lives and is the most important soil layer for pH adjustment.
Manage the soil pH as acidic as possible for the turf you are growing (see Table 1). All but one centipedegrass sample with take-all disease that has come through the MSU Plant Diagnostic Lab has had a soil pH very near 6.0. The adaptive pH range for centipedegrass is 4.5–6.0. The one exception had low levels of potassium and high levels of other nutrients.
Two different techniques have been used to adjust pH: top-dressing with peat moss and using elemental sulfur to drive down the soil pH.
Sphagnum peat moss is used to top-dress areas of lawn where the disease is active. Cover the stolons and any exposed roots. The sphagnum peat moss probably lowers the pH in the immediate area of the stolons and plant crowns so that the disease is suppressed.


Figure 3a-3b. Diseased and rotting roots and stolons caused by an ERI. These samples were easily pulled from the browning patches. Note the lack of roots and broken stolons. Diseased patches have little hold to the soil.
Elemental sulfur (flowers of sulfur or ground sulfur, such as Southern Ag Black Snake Sulfur) is used in two applications 3–4 months apart to drive down the pH of the soil. The label gives the amount to use and other application instructions.
Consult soil test results to make sure nitrogen, potassium and phosphorus levels are balanced. Correct if needed. Lack of potassium makes bermudagrass decline more severe. If you must add a fertilizer, use an acidifying form, such as ammonium sulfate, to keep an acid pH. Avoid nitrate nitrogen. (See Extension Information Sheet 1668 The Plant Doctor: Plant Disease and Fertilization.)
|
Turfgrass |
Mowing Height (Inches) |
Acceptable pH range |
|---|---|---|
|
St. Augustinegrass |
2.5–3.0 |
5.0–6.5 |
|
Centipedegrass |
1.5–2.5 |
4.5–6.0 |
|
Zoysiagrass |
1.0–2.0 |
6.0–6.5 |
Also consult the soil test for calcium levels. In general, plants lacking calcium are more susceptible to disease than plants with enough calcium.
The fungus is known to immobilize soil manganese, but none of the few studies that applied manganese, showed any benefit. Applications may have been too late in the year.
Excessive fertility may also trigger disease, but this is unproven. St. Augustine and centipedegrass were originally selected to thrive in low-fertility areas, and some publicized (high) fertility programs may contribute to this disease.
Mowing height differs with turf type (see Table 1). Besides mowing at the correct height, you should think about how often you mow. Don’t remove more than one-third of the length of the leaf blade in any one mowing. This means you should mow shorter turf areas more often than long areas. Changing to lower mowing heights often triggers the disease, and sometimes just raising the mowing height will greatly reduce symptoms. Frequent mowing at the proper height may very well improve the turf.
Soil compaction limits movement of air into the soil, increases the level of carbon dioxide, reduces soil moisture, and increases runoff. It is harder for turf plants to grow roots in compacted soil. Aerate the turf as vigorously as the roots will allow. The best way is to use a hollow core aerifier. If the aerifier pulls patches of turf loose, the aerification is too vigorous.
Remove and destroy the cores, and do not fill in the holes. If you apply a topdressing, use a light one that is high in acid organic matter, around 3 percent peat. Some reports say activated composts are beneficial, but others have seen no extra benefit from them. If you apply a fungicide, this is a good time to do so.
Thatch is an intermingled layer of dead grass stems, leaves, and roots between the soil surface and the green turf. It occurs when the turf decays slowly before dying. A thatch layer deeper than about three-fourths of an inch tends to raise the turf crowns and roots out of the soil, exposing them to high and low temperatures and drought. Many harmful fungi also survive in the thatch layer. You should work to lessen thatch. Thatch is most common in zoysiagrass.
Since the fungus infects stolons, rhizomes, and crowns, most of the fungus population is in the thatch and root zone soil. If you are going to replace the lawn, removing the upper several inches of matted turf debris and soil will eliminate most of the fungus that may infect the next turfgrass planting.
Most warm-season lawn grasses are sensitive to different kinds and rates of herbicides. For instance, only a few can be used safely on centipedegrass (consult Extension Publication 1322 Establish and Manage Your Home Lawn). Poorly timed applications, or even rates of a safe material applied to a lawn that is already stressed from other causes, such as by moisture, may result in weakened turf, which is more susceptible to disease. Herbicide damage to turf has been associated with later take-all infections.
Watering is critical to the lawn’s health. Proper watering prevents stress, whereas over- and underwatering cause stress. Overwatering also encourages growth of the pathogen. Water deeply so that water penetrates about 4 inches. Do not water again until the soil starts to dry at this depth—unless your roots are so badly diseased that they do not reach this depth. If this is the case, water again when the soil moisture starts to dry around the root tips. You can push an unpainted wooden dowel into the soil to determine the depth of the soil moisture. Moist soil crumbs stick to the dowel, but dry soil won’t. See Extension Information Sheet 1670 The Plant Doctor: Watering and Plant Disease for more watering information. Figure 4 shows the same lawn under poor watering practice and a year later when well-watered and frequently mowed at the correct height. No fungicides were used.


Figure 4a-4b. The same lawn one year apart. The only known difference is better water and mowing practices.
Fungicides don’t control this disease. They may or may not help by removing some stress. Fungicides are not cheap and probably will not work if you have not relieved significant stress factors. Recent research by North Carolina State University indicates the most effective fungicides, regardless of pathogen identity, are members of two fungicide groups: Fungicide Resistance Action Committee (FRAC) groups 3 and 11.
FRAC group 11 contains the strobilurin group whose common names end with “strobin.” An example is azoxystrobin, sold under the trade name of Scotts DiseaseEx Lawn Fungicide and Heritage G. Heritage G can be purchased and used by residential owners, since it is not a restricted-use fungicide. Because this product is mostly used by professionals, it is not generally carried by garden stores and must be ordered. You will need to ask your garden center to purchase this for you. When using it, refer to the home owner portion of the label.
Scotts DiseaseEx is sold in 10-pound bags and Heritage G comes in 30-pound bags. Scotts Disease Ex is applied at 4 pounds per 1000 square feet at 14 to 28-day intervals. Heritage may be applied at 2 to 4 pounds of the product per 1,000 square feet. The label recommends applying twice in the spring, 28 days apart, and twice again in the fall.
Common residential fungicides in FRAC group 3 include propiconazole and myclobutanil. The fungicides in this group which are labeled for residential use tend to “burn” southern turf types growing in hot conditions. Using them may well create more of a stress problem than they are likely to help.
- Myclobutanil. Sold as Fertilome F Stop (0.39% granular, 10 and 20-pound bag), Fertilome F-Stop Lawn and Garden Fungicide (liquid and Ready to Use), Monterey Fungi-Max Brand (liquid), and Spectracide Immunox Multi-Purpose Fungicide Spray Concentrate (1.56% liquid).
- Propiconazole. Sold as Bioadvanced Fungus Control for Lawns (0.51% granular or a 2.42% ready-to-spray), Bonide Infuse Systemic Disease Control (1.8% liquid or ready-to-spray), and Fertilome Liquid Systemic (1.55% liquid).
When applying fungicides, please read and follow the label carefully. For instance, if the label suggests a quick watering (about 1/8”) after application, then do so. It may greatly influence application success.
Since take-all symptoms generally appear in the fall, fungicide applications were historically made during September and October. Now that we have growth rates for the pathogens at various temperatures, we think that the pathogens were actively growing and causing damage earlier in the year. Because of that, the September-October fungicide application timing was too late to stop damage. The fungi were growing minimally, and were not very exposed to the fungicide.
Several studies have now looked at various fungicide application dates and related those dates to soil temperatures at the 2 to 4-in. soil depth. It appears that fungicide applications in central Mississippi would be most effective around May through September, when soil temperature at the 2 to 4 in. soil depth reaches about 75° F.
Many Mississippi residents can access good weather station data near them at http://deltaweather.extension.msstate.edu/stations. Not all weather stations have soil thermometers, but many do.
Find your nearest weather station and locate their 30-Days Table. Scroll to the right to find the temperature readings at the 2-inch depth that occurred during the day. There is a maximum temperature and a minimum temperature reading for each day. The third and fourth columns are the time at which the maximum and minimum temperatures occurred. These are followed by a fifth column, “Soil Temperature Observed at 2-inch depth.” This is an instantaneous reading of 2-inch soil temperature at 7 a.m. standard time. The temperatures from this column should work as well as the minimum morning soil temperature.
If you live in an urban area, your soil temperatures are likely to be slightly warmer than those recorded by these weather stations.
The information given here is for educational purposes only. References to commercial products, trade names, or suppliers are made with the understanding that no endorsement is implied and that no discrimination against other products or suppliers is intended.
Publication 2384 (POD-04-22)
By Dr. Alan Henn, Extension Professor, Plant Pathology.
The Mississippi State University Extension Service is working to ensure all web content is accessible to all users. If you need assistance accessing any of our content, please email the webteam or call 662-325-2262.







